Communication
The Latest Developments & Updates
Find more news on communication-related issues important for the Dutch LSH sector. Ranging from expansion of the Top Sector LSH and important publications.
Health~Holland adopts Zorginnovatie.nl platform
Read more ›

Minister Bruins: "Fund of 105 million euros for promising care"
Read more ›

AMC and VUmc join forces as Amsterdam UMC
Read more ›
Key stakeholders in the development of new antibiotics
Read more ›
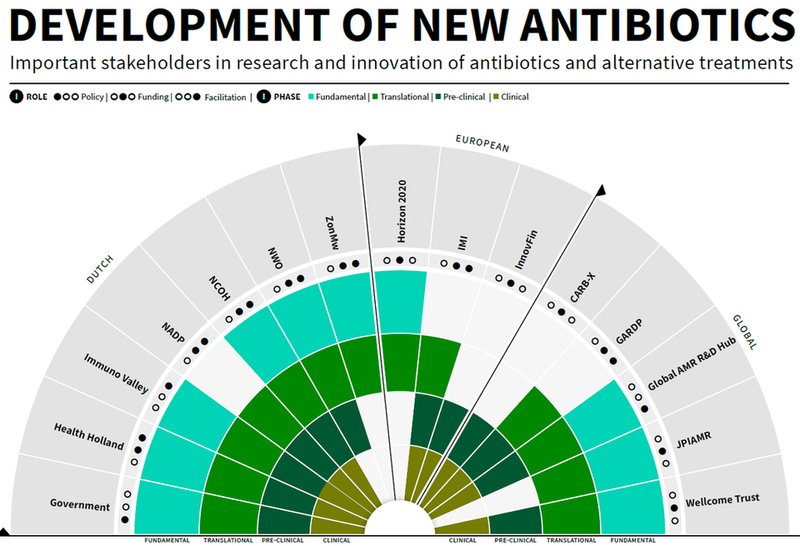
An overview of key stakeholders in new antibiotics
Antimicrobial resistance (AMR) is a growing problem and many parties are involved in tackling it. The Ministry of Health, Welfare and Sport has therefore produced an interactive infographic to provide a better overview of national and international stakeholders involved in the research and development of antibiotics and alternative treatments.
The infographic illustrates the national key players, such as the funding organisations ZonMw, NWO and the Top Sector LSH as equally large public-private partnerships such as the Netherlands Centre for One Health (NCOH) and the Netherlands Antibiotic Development Platform (NADP). Across our borders, European innovation programmes such as IMI and Horizon2020 accelerate AMR-related research lines. And globally, the World Health Organisation (WHO) and the Drugs for Neglected Diseases Initiative (DNDi) established a Global Antibiotic Research & Development Partnership (GARDP) in May 2016. The infographic on this topic provides politicians and decision-makers with a rapid overview of this R&I area.
Open the AMR infographic
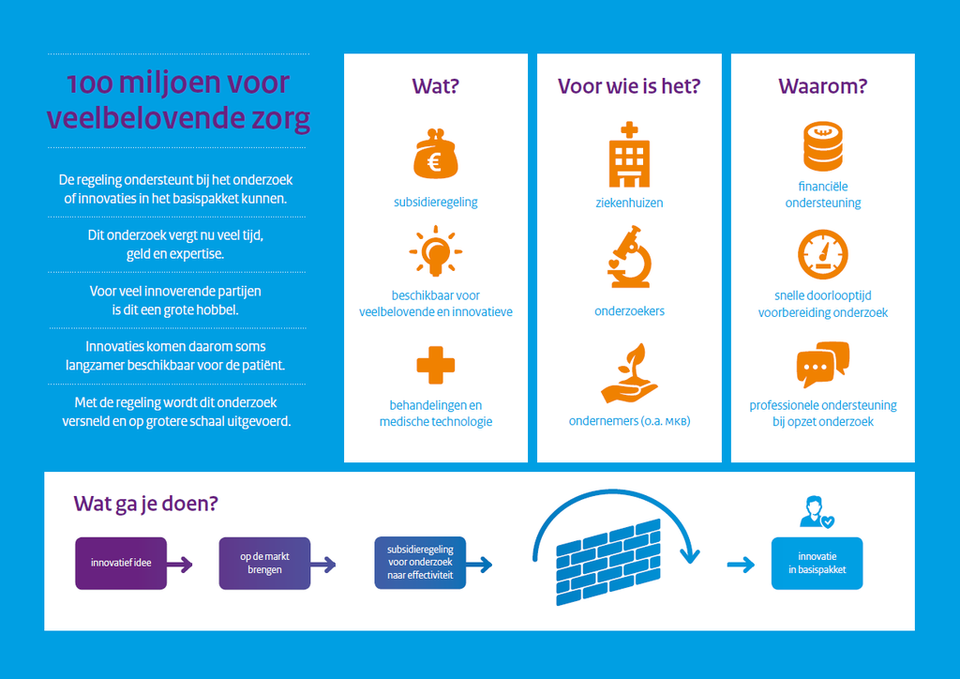
Minister Bruins: "Fund of 105 million euros for promising care"
Making promising treatments, medical technology and medicines more readily available for the patient is the aim of a fund that Minister Bruno Bruins (Medical Care) presented on 22 May. The minister will make 105 million euros available for the fund. With this money developers of new care ideas can be helped to set up and carry out scientific research.
Grant scheme promising care
The total amount benefits three schemes. The first scheme is a grant scheme specifically intended for new treatments, medical aids and medical technology that are generally already admitted to the market, but are not yet reimbursed. Before a new type of care can be reimbursed from the basic package, proof is required that it also has added value compared to existing treatments. Such research is often costly, requires a lot of expertise and lasts for several years. With the money that Bruins has made available for this, developers such as small and medium-sized businesses, hospitals and researchers will be able to pay for this research and the care costs incurred will be covered during the research. The grant recipients will also receive professional help with the design of the research.
Bruno Bruins: "All too often we see that good care ideas get stuck in the development phase and do not end up with the patient."
Bruno Bruins: "All too often we see that good care ideas get stuck in the development phase and do not end up with the patient. The research to bridge the final step towards the basic package and therefore to the patient is complicated, expensive and time-consuming. Especially for smaller companies, individual researchers or hospitals, this is reason to drop out. That is really a shame and bad news for the patients who might benefit from the new type of care. With this new scheme, the last important final step can be made."
This scheme will start on 1 January 2019 and will replace the current conditional admission scheme that has existed since 2012. The eligibility rules for conditional admission proved to be too difficult in practice and so little use was made of this scheme. In consultation with healthcare parties, it was decided to make a new and more accessible arrangement. It is expected that four to five times more use will be made of the new scheme than the old one. As the new scheme is simpler, the total preparation and investigation time required is reduced from about six to about four years.
Medicines and evaluation of existing care
In addition to the grant scheme for treatments and medical technology, Bruins is also working on a similar scheme for medicines. This will be a separate scheme due to the different regulations that medicines are subject to. It will be worked out over the next few months and be presented before the end of the year. The same applies to the scheme intended for evaluating existing forms of care to determine whether these really do work as well as we think. Furthermore, in the negotiation agreement for medical specialist care it has been agreed to evaluate existing care more actively.
Bruins: "Good research into new forms of care is really important. And the same is true for care that we may have been using for years. Does it actually do what we expect? Or do we have to switch to something new? This also merits careful consideration. We do not want unnecessary care or care that does not work in our basic package. This does not benefit patients and we can spend the money concerned far better."
Health~Holland and ZorgInnovatie.nl join forces
At the start of July 2018, Top Sector LSH (Health~Holland) further expanded its portfolio: we have adopted the online platform ZorgInnovatie.nl. ZorgInnovatie.nl originated several years ago from a collaboration between regional partners who were committed to accelerating Dutch innovation initiatives. Now with 700 innovations and over 3000 innovators online, Zorginnovatie.nl has grown into a leading co-creation platform within the healthcare sector.
Connecting people and accelerating scaling up of innovations
ZorgInnovatie.nl is an open platform and community. Everyone can share ideas and contribute to the development of other ideas. It therefore connects existing structures and innovation networks by stimulating knowledge-sharing and intensive collaboration with the aim of connecting and accelerating the scaling up of innovations.
Many healthcare institutions are looking for new ideas to innovate, either to support them in their daily work or because they want to improve their care. Conversely, entrepreneurs or startups are looking for partners. However, high-tech entrepreneurs with a great eHealth solution may lack the necessary contacts in healthcare. And vice versa: healthcare institutions want to bring technology partners into their network to support them to innovate. Financing is often a showstopper as well even though that does not have to be the case. ZorgInnovatie helps to bring this supply and demand together through an online platform and active matchmaking.
Annual challenges to strengthen the goal
Besides exposure through the website, ZorgInnovatie also has two annual challenges to accelerate upscaling. The Sustainable Healthcare Challenge for young professionals with an idea in the early stages of development and the Nationale Zorginnovatieprijs for the most innovative company in the scaling up phase. These are both aimed at companies that have already tested and launched their innovation, but can still use a boost to make their company a real success.
A powerful collaboration
“Vital functioning citizens in a healthy economy” is what Health~Holland aims for. Health~Holland strengthens its mission with ZorgInnovatie. Where Health~Holland has the means and the (inter)national network to scale up innovations faster, ZorgInnovatie.nl provides access to an extensive innovation database and insight into national and regional developments within the healthcare sector. A win-win situation for both organisations. Health~Holland and Zorginnovatie are convinced that their collaboration offers many opportunities for the further development of the platform thereby increasing its value for patients, innovators, care institutions and other healthcare initiatives.
Visit ZorgInnovatie.nl

AMC and VUmc join forces as Amsterdam UMC
The Academic Medical Center (AMC) and the VU University Medical Center (VUmc) joined forces in an administrative merger. The chairmen of both Boards of Directors signed the merger into force this afternoon.
Following an intensive multi-year planning process, the two Amsterdam academic hospitals are working together as of this moment under a collective name: Amsterdam UMC. This enables both Amsterdam university medical centers to further develop their core business together: complex patient care, scientific research, and education & training. Amsterdam UMC kickstarts a gradual process of continuous integration. Initially, this will be most visible in two sectors: patient care and scientific research.
Management
From a legal perspective, AMC and VUmc will remain separate entities. The university medical centers will retain their own employee agreements and supplier contracts. Each university medical center will retain its own Board of Directors.
Patient care
The thinking behind the merger is that Amsterdam UMC will attain an even higher quality of patient care for present and future generations. This will be achieved by clustering specific patient groups in one of the two locations. This enables Amsterdam UMC to safeguard sustainable access to complex patient care.
Scientific research
Amsterdam UMC is already home to eight integrated research institutes (visit amsterdamresearch.org). By joining forces, these research institutes can organise their scientific research more efficiently and they can become a more attractive partner for large-scale, international and multi-year study all over the world. This is an interesting development for students and researchers, both within and outside the country.
Education & training
The separate medical degree courses will co-exist as they are linked to Amsterdam’s two universities: the University of Amsterdam and the VU University of Amsterdam. The three-year bachelor programmes will remain totally separate. The master programmess for student interns – the junior doctor phase – will be adapted in the course of time; once medical specialisations are concentrated at one of the two locations, the intern programme in question for both degree courses will only be offered at that location.
Source: AMC